Knowing battery basics and technologies empowers battery choices.
Aboard any boat with an electrical system there exists a need to store electricity. Enter the battery. But unfortunately (or fortunately), a battery just isn’t a battery, and choosing the type of battery that’s right for you, for your electrical system, and for the way you use your boat, requires an understanding of the different types of batteries on the market today and the advantages and drawbacks of each.
Without delving too deeply into the fine points of battery chemistry or reviewing differences in manufactured quality, following is some information and explanation to help navigate the battery waters.
Batteries 101
Lead alloy plates combined with a sulfuric acid/water electrolyte form the basis of the most common type of rechargeable battery in use today (and for the past 100 years): the lead acid battery. But variations of the lead acid battery have come about as engineers have worked to overcome the chemistry’s most common shortcomings. Accordingly, today there are several subsets of lead acid batteries: the traditional flooded lead acid battery, the AGM (absorbed glass mat) battery, the gelled electrolyte (or gel) battery, and, most recently, the carbon foam battery.
The other battery type on the market today—lithium iron phosphate, or LiFePO4—uses an entirely different chemistry.
All battery types offer significant advantages and disadvantages for the boater. Before learning about the pros and cons of each, it’s important to first take a minute to understand two problems inherent to lead acid batteries: sulfation and stratification.
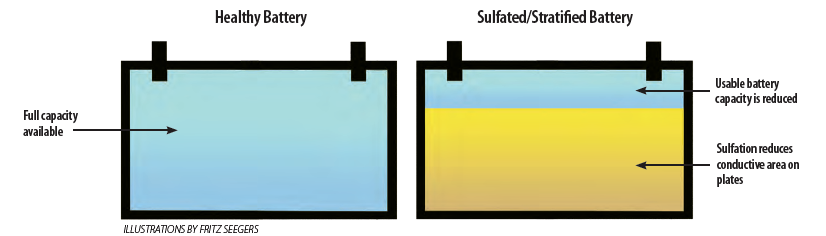
As a battery discharges, small sulfate crystals form. During the next charging cycle, these crystals dissolve back into the acid electrolyte. This process is normal. However, if the charging cycle that follows a discharge cycle is not complete (common when a boat is away from shorepower) only some crystals dissolve, and those that remain can harden and become permanent. With subsequent partial charging cycles, the condition worsens. This is called sulfation. Ultimately, sulfation decreases the amount of chemical available for the necessary reaction and the amount of surface area on the lead plates for that reaction to occur, thus limiting the battery capacity.
Stratification is the tendency for the denser high-sulfate electrolyte (in a water-acid mixture inside a battery) to concentrate near the bottom of the battery as a result of gravity and lack of mixing. Stratification can exacerbate sulfation. Both problems can be addressed with a procedure called equalization; see “All Things Being Equal” beginning on page 22.
Regardless of sulfation and stratification, every cycle causes some wear to a lead acid battery, the severity of which depends on the depth of discharge, time to the next recharge, thoroughness of the next recharge, design category of the battery, and manufacturer quality and design details.
Batteries are also slowly damaged by corrosion on the lead plates. Overcharging is a primary culprit, but no matter how carefully the battery is maintained, every battery design has a finite life, after which the plates will simply fall apart. For boats that spend a few nights at anchor, and for starting batteries, internal corrosion is often the limiting factor.
Finally, any battery type can be wrecked by misuse or poor maintenance. All lead-type batteries can be ruined by being left fully discharged for a few months. All it takes is a small load that is overlooked and no charging source to compensate. Overcharging increases corrosion and can boil out the electrolyte. Lithium batteries can be ruined by overcharging or charging at subfreezing temperatures. Premium batteries will only deliver full value if maintained properly.
With a general understanding of the inherent problems that afflict batteries, let’s take a closer look at considerations for each battery type.
Flooded Lead Acid (FLA) Batteries
These are the traditional electron-storage workhorses of cars, trucks, planes, and boats. They come in different sizes, usually the bigger the battery the more energy it stores. They come in 6-volt and 12-volt configurations, with thin lead plates (starter batteries) and thicker lead plates (deep cycle batteries), sealed and maintenance-free, or with caps that allow for lost battery acid to be replenished.
Negatives:
- The deeper a flooded lead acid battery is discharged (reflected as a percentage of total capacity in amp hours), the harder it is on a battery. A flooded lead acid battery will tolerate only a finite number of discharges in its life (the deeper, the fewer). For reasonable life expectancy, manufacturers advise that flooded lead acid batteries should not be discharged below 50 percent state of charge (SOC) and then should be fully recharged within a day or so. Practically, this means that only half a battery bank’s rated capacity is available for use. If the battery is commonly only 85 percent recharged (the result of a diminishing acceptance rate near the end of the charging cycle) then only one third of rated capacity is available. In other words, up to three times the cost must be spent and three times the weight carried aboard.
- Compared to how quickly they can be discharged, it takes a long time to recharge flooded lead acid batteries. This is because as flooded lead acid battery voltage increases, the charge acceptance rate decreases. So while it’s easy to pump a lot of energy into a battery that’s been discharged to 50 percent, once the battery returns to about an 80 percent state of charge, the amount of current it will accept drops considerably. After about 95 percent, the current acceptance rate drops even more. So, it may take nearly as long to charge from 95 percent charge to 100 percent as it did to bulk charge from 50 percent to 95 percent. Is it any wonder that boats untethered from shorepower often fail to fully recharge, thus increasing sulfation and reducing battery life?
- A traditional marine battery requires maintenance. This includes routinely replenishing water, keeping the terminal connections clean and corrosion-free, and regularly equalizing to minimize the effects of sulfation and stratification.
- Batteries built with lead are heavy. Aside from the drawbacks of concentrating weight aboard, they need to be sturdily and firmly secured aboard a vessel that rolls, pitches, and could possibly invert.
- Flooded batteries produce oxygen and hydrogen when being charged, so they need adequate ventilation where they are installed.
- Flooded batteries can spill. Battery boxes must be able to contain spilled acid. They should not be located near or below equipment that is vulnerable to corrosion.
- Efficiency and capacity will diminish as the battery ages.
Positives:
- A well-built, deep cycle flooded lead acid battery is rugged, widely available, and relatively inexpensive.
- You can expect a lifespan of 200-600 cycles from a run-of-the-mill deep cycle battery, or as many as 2,000 cycles from a well-maintained golf cart battery, such as the popular Trojan T-105s. Starting with a large enough battery bank and creating an environment in which it isn’t discharged too deeply, is regularly returned to a full charge, and is maintained properly can go a long way to stretching the lifespan of a flooded lead acid battery. The 50 percent maximum discharge is a fuzzy number. A large bank that is recharged at only 20-30 percent discharge will last longer, be twice as heavy, and cost twice as much. A smaller bank, frequently discharged to 35 percent, might be 30 percent cheaper and lighter, but only lasts one third as long for the full-time cruiser. It also provides less reserve capacity for long nights and eventual loss in capacity. On the other hand, the weekend cruiser might not see a huge difference in lifespan, since the number of cycles is low and the battery will most likely die from either corrosion or a maintenance error rather than high cycles.
Absorbed Glass Mat (AGM)
AGM batteries are lead acid batteries, but instead of lead plates submerged in acid, they’re built of lead plates separated by thin fiberglass mats saturated in battery acid. The plates and mats are packed together very tightly, and the battery case is sealed. There are AGM starting batteries and AGM deep cycle batteries. AGM batteries are optimal when they can be quickly and fully recharged: marina hopping, day sailing, and boats with substantial charging resources.
Negatives:
- AGM batteries are particularly vulnerable to sulfation if not brought back to full charge within a day or so (recharge to 80 percent of charge is not sufficient). AGM batteries do not respond as well to equalization as a well-built deep cycle flooded lead acid battery, and some cannot be equalized at all.
- AGM batteries are more expensive than flooded batteries.
- Just as a flooded lead acid battery, these batteries are built with lead and are heavy, with all the issues that weight brings.
- Efficiency and capacity will diminish as the battery ages.
Positives
- Compared to flooded lead acid batteries, AGM batteries accept relatively high recharge currents (they can be charged more quickly) because they have a lower internal resistance.
- AGM batteries are completely sealed and can be mounted in any configuration, even on their sides. In normal use, they don’t vent.
- AGM batteries are not as susceptible to stratification.
- Other than equalization for select AGM batteries, and keeping tabs on terminal corrosion, no maintenance is required.
Gelled Electrolyte (Gel)
Gel batteries are constructed similarly to flooded batteries, but with a silica added to the battery acid, turning it to a thick, gelled substance. The best application for gel batteries is a smaller bank that will be cycled deeply and frequently, possibly without the availability of timely recharge.
Negatives:
- While gel batteries are a bit more resistant to the ill effects of deep discharges, they are less able to recover from sulfation as they cannot be equalized.
- Gel batteries are more expensive than flooded batteries.
- Just as the previous two types, they’re heavy.
Positives:
- Gel batteries are highly resistant to stratification.
- Compared to similar flooded or AGM batteries, gel batteries can tolerate a higher number of deep discharge cycles.
- Though efficiency and capacity will diminish with age and use, the effects tend to appear rapidly at the end of a battery’s life, rather than gradually over time.
- Gel batteries are completely sealed and can be mounted in any configuration, and in normal use, they don’t vent.
Carbon Foam
Firefly International Energy Company holds the patent on this relatively new technology: a lead acid battery in which much of the lead is replaced by a carbon foam material. The construction is similar to AGM batteries but with significant advantages over AGM, gel, and flooded lead acid batteries.
Negatives:
- More expensive than all other lead acid-type batteries. That said, if the advantages are as reported, including longer lifespan, they may warrant the higher price tag. One internet forum commenter called carbon foam batteries the poor-man’s lithium batteries.
- The technology has been around for a relatively short period of time, so many claims of longer lifespan (compared to flooded, AGM, and gel batteries) have not been empirically proven aboard many boats.
- Despite the carbon foam material used to replace much of the lead on the negative plates, these batteries are no lighter than conventional AGM batteries, and so have the same weight issues.
Positives:
- The carbon foam material resists corrosion and sulfation and provides a much greater surface area (due to the honeycomb shape of its cells) than the pure lead plates. Accordingly, these batteries are highly resistant to damage from being stored at low charge states.
- Because these batteries tolerate deeper discharges and sustained under-charged states, ostensibly a smaller bank can meet the same power needs, meaning less weight and perhaps comparable cost to other AGM options.
- Carbon foam batteries are completely sealed and can be mounted in any configuration, and they don’t vent in normal use.
- Carbon foam batteries have less internal resistance than the best conventional AGM batteries and thus accept even higher recharge currents (they can be charged more quickly).
- Carbon foam batteries are not as susceptible to stratification.
Lithium Iron Phosphate (LiFePO4)
There are many lithium-ion battery chemistries on the market in batteries powering laptops, phones, cars, and more. But this particular chemistry—lithium iron phosphate, first described by researchers at the University of Texas in 1996—offers thermal and chemical stability that is superior to all the others. That means it’s safest.
I’ve received enough glowing reports that it’s about time to recommend LiFePO4 for widespread use in high-cycle applications. Heck, Toyota made the switch for their Prius batteries in 2015. However, there are some caveats. Consider a sudden house-bank failure like I faced a few years ago, the result of my misjudging battery health before casting off on a spring cruise. I made a quick phone call to a local chandlery and had a replacement set of group 27 flooded lead acid batteries delivered dockside within hours. Had I been running LiFePO4 batteries at the time, I’d have waited a week and spent thousands. In my case, the bill was a few hundred dollars, replacement took less than an hour, and we were on our way the next day.
Negatives:
- The first thing that will strike you is the price, at nearly 4 times that of premium lead acid batteries. Even so, their proponents, including a good many long-term cruisers, believe they are the most economical long-term solution for heavy-duty users.
- Regulation of charging is very different from lead acid. Part of the expense is a complex battery management system (BMS). Because even a slight imbalance between cells can lead to dangerous overheating problems, control systems are built into the consumer-packaged batteries (Battle Born, Lithionics, Mastervolt, Relion, Trojan, Victron, and Valence provide these systems). Marketed to sailors as drop-in replacements, these may not be the best route to go. Lithium batteries are better left slightly below full, but all of the smart chargers on your boat will take them to float and leave them there. To get the full value of rapid charging and long life you will need an integrated management system installed for another $1,000 to $1,500, and you will want to work with a qualified installer, following factory guidance.
- I recommend having a good talk with a lithium battery specialist before buying any drop-in system as a replacement for an existing lead acid battery bank, because they aren’t the same thing. Do not buy naked cells (without a built-in BMS) off the internet and try to build your own system; this is a good route to either short battery life or a fire. Carbon foam has some similarities (fast charging, partial state of charge tolerance) and is generally a better drop-in replacement for FLA for most sailors, particularly if weight is not critical.
- Cold weather charging is a potential Achilles’ heel. Lithium batteries can be permanently and severely damaged by just a few charging cycles at near-freezing temperatures. If the battery can be disconnected for winter storage, self-discharge rates are very low and the battery should be fine in the spring. However, if the battery must be kept online because of sump pump demands, the situation gets hairy. It’s simple enough to provide charging through shorepower or solar panels, but the controller must disable charging below about 40° F and disconnect the battery from the load. I find this a little frightening for boats stored in the water through the winter. The normal solution is installing a lead acid battery to handle sump pump demands. Automobiles with lithium batteries use built-in heaters.
Positives:
- Very low weight compared to lead acid batteries. This difference may be especially appreciated aboard multihulls
- LiFePO4 batteries have an exceptionally long cycle life, they recharge more efficiently, they do not require immediate full recharge, and they deliver more consistent voltage during the discharge cycle.
- Because they operate well at a state of partial charge—in fact, they like it better than being fully charged—a battery bank can be about a third smaller in terms of amp-hours, taking some of the sting out of the price differential.
Weighing the Information
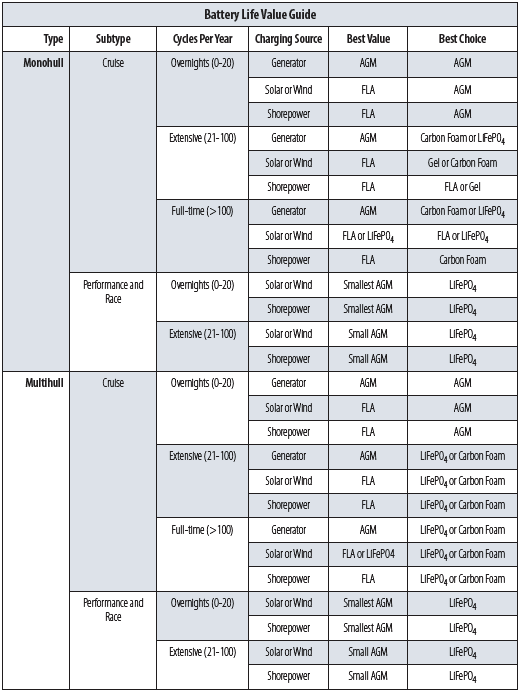
Just as every boat is a compromise, so is every battery type. But which compromises best align with your anticipated use? Ask yourself how often you foresee sleeping on the hook over the next five years. How much does battery weight matter to you? Will you charge your batteries rapidly with a generator, or will you push the electrons in more gently with solar power? Do you have enough charging capacity to fully recharge the battery within a day or so of a deep discharge? How much reserve capacity do you need? Are you motivated to properly monitor your battery system and tweak a full life expectancy from a premium product, or do you suspect you’ll make mistakes and prefer the premature replacement that doesn’t break the bank?
Weight is a good place to start. What is the cost of carrying an extra pound for the life of the battery? It’s easy for the heavy cruiser to say that weight and performance are unimportant, but a performance trimaran or planing sport boat will find 100 pounds unacceptable and 50 pounds unpleasant. Reducing weight is likely important for windlass and bow thruster batteries mounted forward.
And if weight is critical, consider that weight can be saved by reducing the size of the battery bank within the same battery type. For example, if you’re regularly discharging a lead acid battery below 60 percent state of charge, conventional wisdom would indicate it’s time to increase the size of the battery so that its life isn’t reduced. How important is this reduction in lifespan? The batteries on a boat that is very seldom anchored out will die of corrosion and old age before the cycle life becomes important. On the other hand, the long-distance cruiser may be irritated when batteries fail within a few years. A racer may not mind dropping in a cheap battery every few years if it saves 100 pounds.
Reserve capacity is also reduced by using a smaller bank. Some battery technologies—principally lithium and carbon foam—better tolerate deep discharges, but if your design basis includes discharging a battery 80 percent, you have sacrificed a safety margin with regard to reserve power availability.
Is your auxiliary an outboard motor with a typical tiny alternator? Don’t bother with the complexity and expense of a separate starter battery and house bank. Connect the outboard starter to the house bank. On the odd occasion you inadvertently run the battery flat, use the pull-cord to start the outboard. With one bank, charging and usage are more equal, house bank capacity is increased, and battery life is improved.
Much is made of the ability of AGMs, Gels, and LiFePO4 to withstand flooding and knockdowns, but how important is that feature? Any battery, regardless of type, must be robustly secured to avoid risk of damage and electrical shorts. And how much does a flooded lead acid battery leak when it’s inverted for a short time? The caps will remain in place, and more than a few cars have rolled over without losing more than a few drops. If the boat is flooded to the depth where battery acid is likely to float out of the cells, it will emerge only very slowly, to be diluted by saltwater.
All of these battery types can provide good value and reliable operation. Size the bank appropriately, avoid excessive discharge, and recharge in a timely manner with a smart regulator (and a regulator matched to the battery type). Occasional and even frequent weekend cruisers are often happy with inexpensive deep cycle batteries. If they make a mistake and ruin the bank, it’s not a big deal. Others prefer AGMs because they don’t have to check the water.
Full-time cruisers like the high cycle life and robust durability provided by golf cart batteries, but these batteries do require attention. Early adopters have been thrilled with carbon foam and LiFePO4 batteries; more efficient charging by solar or generator is a strong point if you’re living on the hook, and either should withstand over eight years of daily use. Racers and performance boat owners like either reduced-size AGM banks or LiFePO4, depending on whether their heart beats for throwaway simplicity or high tech.
My personal favorite? It depends on which boat you ask me about. I liked LiFePO4 in my Kevlar speedster (racing and a few overnights), flooded lead acid in my cruising cat (extensive cruising), and a single AGM in my trimaran (day sailing and a few overnights). Horses for courses.
A Word of Warning About Undersized Banks – DF
Manufacture life expectancy versus depth of discharge tables are misleading in two critical ways. First, the test protocol requires that the batteries are fully recharged after each cycle, a practice that extends the life of lead-acid batteries by minimizing sulfation. In the real world of the cruiser, getting back to 85 percent charge is good, and sulfation will progress faster than the charts suggest.
Second, battery life always declines with age and the corrosion that goes with it, no matter how well a battery is cared for. If you start with a bank sized with 30-40 percent average discharge in mind, with occasional dips to 50-60 percent discharge, within a few years that will become 40-50 percent average discharge with occasional dips to 70 percent discharge. That’s workable. On the other hand, if you designed the bank for 40-50 percent average discharge, that will soon be 60-70 percent discharge with dips to 80 percent discharge, and the bank will be spent in just a few years. Design conservatively with aging in mind. Only a racer will be happy with cutting it thin.
Battery Sizes – Michael Robertson
At the Annapolis sailboat show this past October, a few of us got to wondering about battery sizes. We each acknowledged that 8D batteries were big, and we could identify them by sight. We agreed that group 27 batteries were just a bit smaller than group 31 batteries, and we could identify them too. But some of us swore that not all group 27 batteries were the same size (nor were all 8D and group 31 batteries the same size) and others of us believed they had to be. A quick search of specific battery dimensions online confirmed that sizes varied. What was going on?
Being at the Annapolis show, I cornered Nigel Calder for the definitive answer. Nigel didn’t even have to stop to think: “Group numbers refer to battery sizes and the specific dimensions are specified by Battery Council International.” Got it. A quick check online turned up a chart of BCI group numbers. Indeed, for each battery group (there are 81 groups listed!) there are specific length/width/height dimensions, in millimeters and inches—exact measurements. But we noticed a couple things that explained the variable sizes among manufacturers. First, the BCI dimensions are maximum dimensions. Second, there are so many groups that some of them are broken into subcategories, each with a slightly different dimension. For example, a group 27 battery should measure no more than 121?16 x 613?16 x 87?8, but there are group 27F and group 27H dimensions that are slightly different.
Starter Batteries Versus Deep Cycle Batteries – Michael Robertson
Flooded lead acid batteries are made with either thin lead plates (starting batteries) or thick lead plates (deep cycle batteries). The thinner the lead plate, the faster power can be released from a battery. For a car battery that delivers a big shot of power to start an engine and then lives in a charged state (getting power from an alternator), a thin-plate starter battery makes sense. But thin plates aren’t as robust as thick ones, and they won’t tolerate repeated long-period, deep discharges typical for boat at anchor, on which lights, fans, and inverters are used for days with limited charging cycles.
Deep cycle batteries, with thicker plates and more electrolyte, are the flooded lead acid batteries used most often in marine battery banks (as well as in golf carts, electric warehouse forklifts, and industrial equipment). This is because the thicker plates are less susceptible to corrosion (the inevitable process in which lead sloughs off plates over time) and can withstand many more deep-discharge cycles over the battery’s lifespan.
Many boats have two battery banks. One contains a starter battery, used strictly to start the engine and then recharged soon after, as in a car. The other is a house bank, comprised of deep cycle batteries used to power lights, radios, inverters, and other things. These two banks should remain isolated from each other, as combining batteries of dissimilar types, sizes, and ages can damage batteries.