A simple, low-cost stovetop heater takes the edge off a chilly cabin.
Few sailboats have a dedicated heating system for warming the cabin, something built-in and properly vented. This makes sense because a cabin heater with a flue can be a complicated, bulky, and expensive affair, and something that’s perhaps used only on occasion. So, when it’s cool and we decide to spend a night swinging on the hook, away from shorepower, many of us rely on portable heaters, usually propane.
A portable propane heater can do the trick, but these heaters also require ventilation as they consume oxygen and emit water vapor and carbon dioxide—and produce carbon monoxide as a byproduct of incomplete combustion. The rough-and-ready solution is to crack a window, but how wide? And then how much of the heat is lost?

There is another approach, a simple, non-bulky, low-cost solution that uses an existing onboard heat source: the stove.
The stove can’t tip and start a fire (unless something combustible falls on it, which can be prevented by maintaining a clear countertop). And although stoves generally don’t include an oxygen depletion sensor like you’d find on a portable heater, it’s easy to add a carbon monoxide monitor to the cabin.
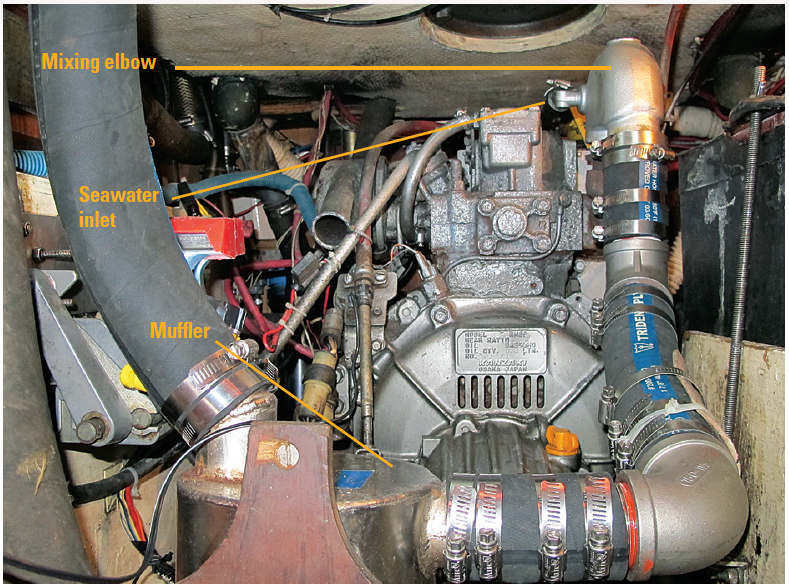
All that’s needed to turn the stove into an effective, ventilated cabin heater is to provide a heat transfer surface and a way to funnel the exhaust outside the cabin. In fact, the ubiquitous Sig Marine (now Dickinson) Cozy Cabin heaters are not much more than this: a simple burner below an inverted-can heat transfer space, connected to a 1-inch stainless steel flue.
The stove aboard my boat is an Origo 2000, a non-pressurized alcohol stove fueled by denatured ethanol. I have turned it into an efficient, safe heater by using a retired, upside-down stainless steel soup pot as the heat transfer surface, resting atop the burner. Here’s how I did it.
Materials
- 1-inch ribbed duct hose, stainless. McMaster Carr 5241K13, $23.80 for 5 feet
- 4-quart stainless soup pot, thrift store or surplus
- Aluminum flashing
- 6 inches of 1-inch copper tubing
- (1) 1-inch copper 90-degree el
- Assorted small screws and two long cotter pins or nails
- Plywood or similar to build slider insert, or better, install the flue through cabin roof or bulkhead if you will use the heater often

Here Is How I Did It
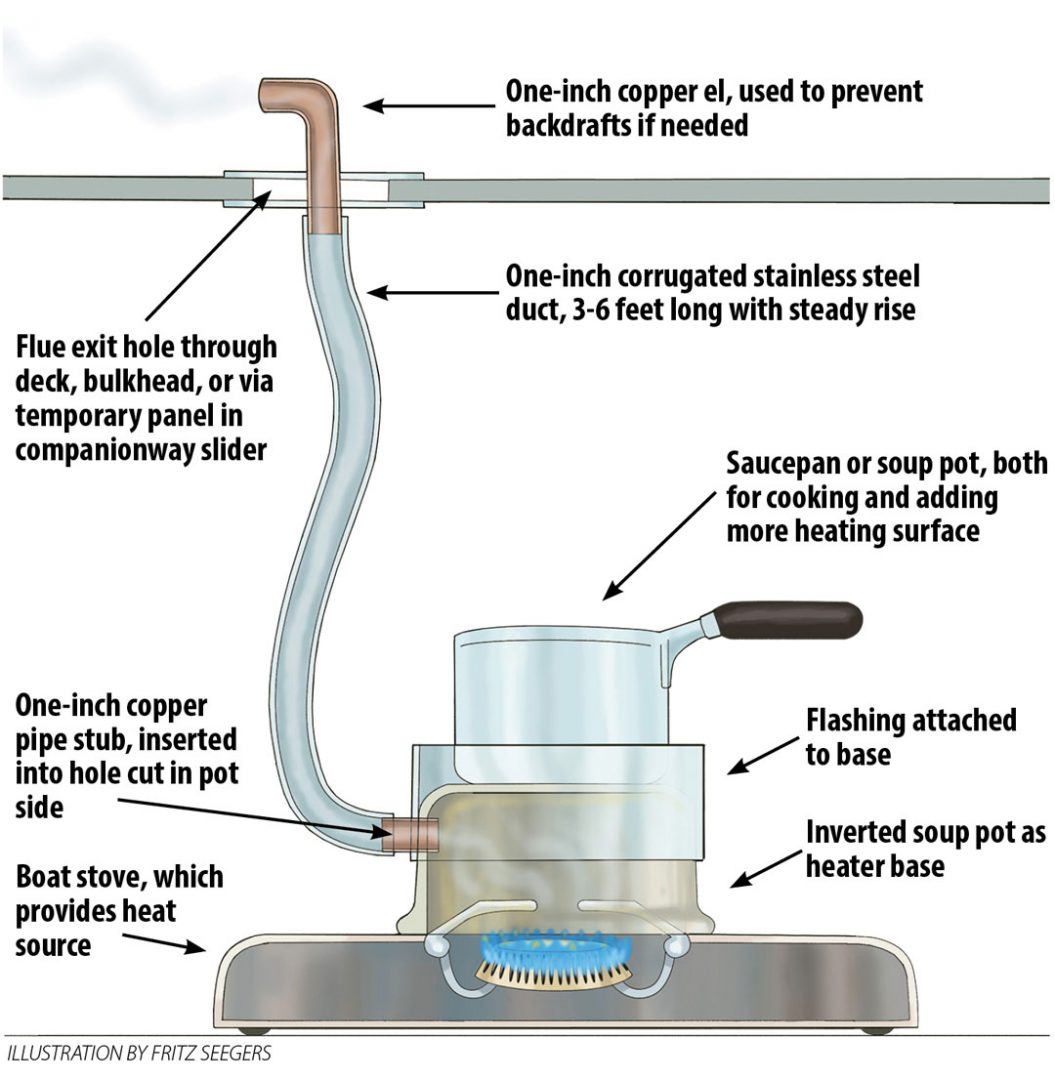
First, I cut a 1-inch flue opening in the side of the pot with a hole saw, into which I inserted a 1-inch copper pipe stub. I attached the pipe by slotting to create tabs inside and out, which I secured with small bolts (blind rivets would have been a cleaner solution). Next, I added a 3-inch-high flashing skirt to accommodate and hold a pot on the top. The skirt also improves the seal around the flue exit.
For the flue, I attached 1-inch (ID) corrugated stainless steel duct to the copper stub on the pot, using a cotter pin driven through both. There is no need to seal this connection because the entire length is under slight suction. This flue must rise steadily, without low spots, and be 3-6 feet long.

The first season I used my heater, I kept the exhaust system simple. I cut a 3-inch-wide board to fit the aft end of the companionway slider, drilled a hole in the board, and passed the exhaust end of the flue through it. This way, I didn’t have to drill a hole in the boat.
But after realizing my heater worked, and that there was nothing to improve, I drilled a hole in the cockpit bulkhead for a permanent flue, which is just 3 inches of 1-inch copper pipe. There’s no need to insulate the pass-through because the flue temperature never exceeds 120° F. So far, rain has not blown in, and I could easily cap it if necessary. If the wind is from ahead or the beam as it would be sailing or at anchor, the draft is fine, but if there’s a strong wind from astern—for example in a marina—it can blow the draft into the cabin. I keep a 1-inch copper 90 el, which, when pointed up, solves that.
The burner on my Origo 2000 stove is rated at 7,000 Btu. Because my stovetop heater uses the same flue size as the 5,000-Btu Sig Marine Cozy Cabin heater, I decided to not exceed that combustion rate and keep the burner valve between 1⁄2 to 2⁄3 open. This will give a heat output of about 1,500 watts, enough to fully warm the cabin of my 24-foot boat in 10-20 minutes in cool to cold weather. If I can feel some of the exhaust backing out from under the inverted pot and into the cabin, I know the burner is firing too high.
To confirm the heater’s efficiency and safety, I took some measurements. I wanted to be sure the exhaust was leaving the cabin, so I scanned the surface and flue with an infrared thermometer. The pot surface reaches about 290-340° F. By the time the exhaust reaches the outlet, it has cooled to 80-120° F, proving high efficiency. I tested the air 1⁄2-inch below the lower rim of the pot, curious whether some of the exhaust was sneaking out under the bottom; the carbon dioxide level was barely above background, and the temperature never exceeded 200° F, confirming that essentially all of the exhaust goes up through the flue.

The purpose of the flashing rim and upper pot is to add heat transfer surface area and improve heating efficiency. Most of the time, the upper pot will be empty. But if I crank the burner to high and add two cups of water to the upper pot, the water will boil in 10-15 minutes. With the burner turned down—as it should be to warm the cabin—it will simmer soup, without burning or polluting the cabin with carbon dioxide.
Another advantage my vented, stovetop heater has over a portable propane heater is dryness. Burning propane (or alcohol) produces water vapor. Dry air is nearly as important as warm air to comfort because insulation remains more efficient. An unvented heater will raise the humidity 30-60 percent, making for a clammy cabin and causing condensation on the windows—not a problem with the vented heater.
This heater will work with stoves burning alcohol, propane, CNG, butane, and kerosene, as long as the firing rate is similar. The differences in combustion are small.
Although the heater is stable enough to be used underway, the pot on top is not. Also, I don’t leave the heater on while I sleep. It’s not designed for that, and I like sleeping under a thick quilt or in a warm sleeping bag. Nor do I leave it on while away from the boat. I run it from sundown, through the dinner hour, until ready for bed. I use a carbon monoxide monitor, and when cooking on the stove, I usually crack the companionway slider to evacuate water vapor.
A stovetop cabin heater sure beats cold fingers or a warm, damp cabin. More importantly, it avoids low oxygen and high carbon dioxide, and minimizes carbon monoxide accumulation risk. 
Just running the Origo stove for one hour with the regulator half open and the cabin tightly closed, carbon dioxide reaches 15,000 ppm and carbon monoxide level reaches 100 ppm, both well into the danger zone. A cracked window will reduce this, but unless the window is open wide enough to admit considerable draft—rather defeating the purpose in cold weather—there remains serious risk of exceeding the limits for good health and clear thinking.
Keeping a Clear Head—DF
I value my brain cells and will not take risks with them; I’ve never been a fan of portable heaters.
Carbon dioxide concentrations over 1,500 ppm affect thinking and are universally considered cause for investigation and correction. Over 2,000 ppm is considered serious by health departments. Ten-thousand ppm is the threshold of serious problems with medium-term exposure (many hours to days), and both OSHA and NIOSH limit workplace exposure to 5,000 ppm.
I did a few simple calculations for my Corsair F-24. If I run an unvented portable propane heater at about 4,000 Btu/hour (what is required for a 45° F evening), assuming no ventilation other than combustion air replacement, carbon dioxide levels will reach 60,000 ppm within six hours, about the threshold of unconsciousness.
The Flowerpot Heater Myth—DF
The story goes like this: Place a flowerpot over a stove burner, and you have an effective, safe cabin heater for peanuts. Thermodynamics says otherwise, but I decided to test this myth anyway, because it would be great if it were true. Unfortunately, none of it is true, except for it being cheap.

Without regard to the efficacy of the flowerpot heater, using one can be dangerous. About 60 percent of glazed pots and 30 percent of unglazed pots I tested shattered violently at 400˚ F, throwing large, hot pieces of pot 2-5 feet. Only by limiting the temperature to 300˚ F (by keeping the flame quite low) was the shattering risk reduced to an acceptable nonviolent level. Considering that even Corningware is not stovetop safe, this should be obvious.
I used small (5- to 6-inch-diameter) and large (7- to 9-inch-diameter) clay flowerpots over a natural gas flame (bear in mind that propane is a little hotter, alcohol a little cooler). I measured pot temperatures and cooling rates, and I calculated radiant heat output of the naked flame and burner grate, and of the pot and burner grate. It’s important to note that when the pot is on the burner, any heat radiated by the naked flame, burner, and grate is blocked by the pot.
First, some basic physics. All heated materials absorb and then radiate heat. The amount they radiate is proportional to the fourth power of their temperature, as measured on an absolute scale, either Rankine or Kelvin. The hotter they are, the more they emit and the shorter the wavelength of the emissions.
Radiation is just one method of heat transfer; the others are conduction (the direct transfer of heat between solid objects in contact with one another, like how a hot pot heats the metal handle attached to it) and convection (the movement of heat energy within a gas or liquid, like how a heat source warms the air near it and that air begins to move and circulate). But it’s only radiant heat that is relevant to the question of whether the flowerpot heater offers a benefit. This is because for conductive heat to offer any benefit, you’d have to hold the pot. Convective heat isn’t very relevant either, because the balance of the heat in the flame would end up mixed in with the cabin air anyway.
A 7-inch flowerpot heated to 320˚ F emits about 150 watts (500 Btu) of thermal radiation. If the pot reaches 400˚ F, the amount of radiation emitted increases to about 370 watts, or about 1,100 Btu. In contrast, a naked propane flame is over 1,500˚ F and the burner grate will reach 400˚ to 800˚ F, depending on location. The radiation rate of the flame and grate alone is greater per unit area than that of the flowerpot, but the area of the flame and grate combo is much smaller. The result is that the pot offers little increase in the amount of heat radiated.
On the other hand, portable propane heaters, like the Mr. Heater line, heat a ceramic grid red hot (about 2-3 times hotter than the flowerpot on an absolute scale), and are thus 20-80 times more efficient at converting fuel energy into radiant heat. However, that does not mean they create more heat; the total Btu is fixed by the amount of fuel burned and cannot be increased.
It’s often said that the heated pot stores and releases heat over a longer period, thus keeping the cabin warm long after the stove is off. This sounds good but isn’t really accurate. First, a large pot will cool relatively quickly, smaller pots even faster. A 9-inch glazed flowerpot that starts at 300° F drops to 195° in five minutes, 130° in 10 minutes, and at a half an hour is at 85° F. The same pot starting at 450° F drops to 345° in five minutes, 280° in 10 minutes, and is at 110° in a half an hour. The hotter the pot, the faster the cooling.
Second, a large pot that weighs 3.2 pounds doesn’t store a lot of heat, a smaller pot even less. The specific heat of brick clay is .22 Btu/pound, and let’s say, for example, that a large pot cools from a high of 450˚ F to a low of 55˚ F overnight. The heat released by the pot is 278 Btu.
By comparison, let’s say the cabin sole, bulkheads, and furniture have all been heated to 70˚ F during a typical day, with the air temperature dropping quickly after sunset. The specific heat of pine (for example) is .66 Btu/pound. Assuming there are 500 pounds of furnishings, linings, flooring, and supplies in the cabin, with an average specific heat value equivalent to pine, the heat capacity of the cabin infrastructure is about 6,600 Btu. Additionally, the cabin furnishings will cool much more slowly, because at any given time, the temperature difference between the furnishings and the cabin air is very small. As noted above, hot objects cool far more rapidly than cooler ones. Thus, the clay pot represents only 4 percent of the heat holding capacity of the cabin as it cools during the evening (and this heat is given up within a few minutes). A rounding error at most.
Bottom Line: If it is merely cool, pull on a sweater. You can run the stove for a few minutes now and then, cooking a meal or making tea. This is less wasteful and just as effective as the flowerpot. If it is actually cold, install a proper heating system. The benefits of a flowerpot on the stove are at least an exaggeration, and more realistically, a dangerous myth.